Import Refusals
NEW!
- Dashboard has been integrated with the OII Unified Logon application. To obtain the credentials necessary to use the API, please submit authorization key requests using the online OII Unified Logon application.
Caveats:
- Certain information in these datasets may not be presented or may have changed since the posting. The datasets are update weekly and only include final actions. If you need to present more recent or more complete data for official purposes or have questions about obtaining other data, please contact the Division of Freedom of Information about what materials may be available in electronic reading rooms or inquire about other datasets that would satisfy your needs.
Refusals by Product Category:
Animal Feed
Cosmetics
Devices
Drugs and Biologics
Housewares & Food Related
Human Foods
Radiological Health
Tobacco Products
All Refusals
Unique Shipment Lines Refused by Country
Number of Import Refusals
- 0
Import Divisions*
Division of Northeast Imports
Division of Northern Border Imports
Division of Southeast Imports
Division of Southwest Imports
Division of West Coast Imports
Import Districts*
Atlanta District
Baltimore District
Chicago District
Cincinnati District
Detroit District
Florida District
Los Angeles District
Minneapolis District
New Orleans District
New England District
New York District
Philadelphia District
San Francisco District
San Juan District
Seattle District
Southwest Import District
FDA Import Offices and Ports of Entry
FDA field offices that process imports are part of the Office of Regulatory Affairs and are divided into 5 Import Divisions. The FDA’s Office of Regulatory Affairs is the lead office for all FDA field activities as well as providing FDA leadership on imports, inspections, and enforcement policy.
Inquiries related to a specific import entry are most appropriately routed to the import division handling the entry. Each FDA import division contains a main office and resident posts, please select the division in which you are making entry and the contact information for the division and resident posts will be listed.
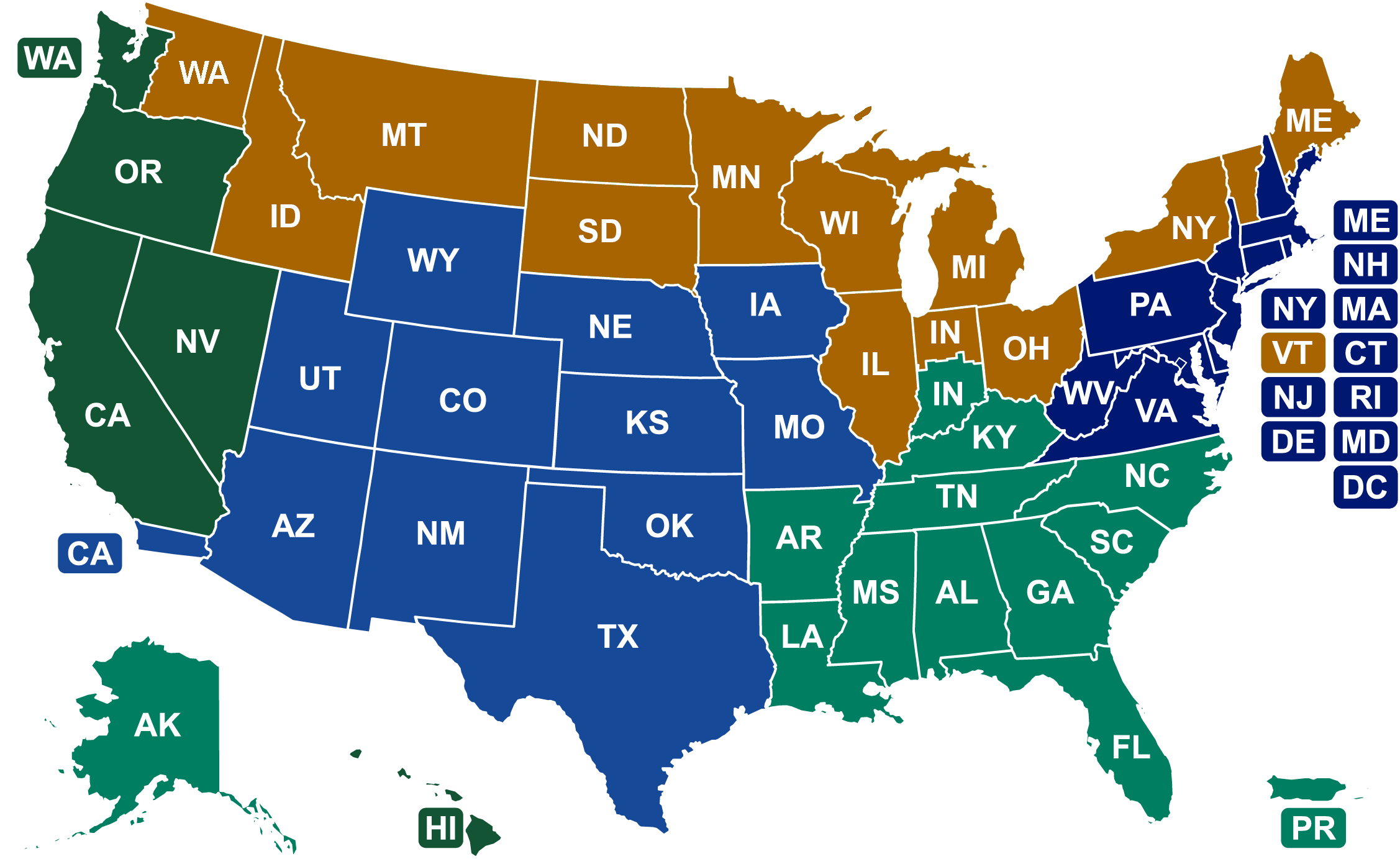

Division of Northern Border Imports (DNBI)
Division of Northeast Imports (DNEI)
Division of West Coast Imports (DWCI)
Division of Southeast Imports (DSEI)
Division of Southwest Imports (DSWI)
Download Dataset
If filters are applied on this page, the ‘Filtered Data’ link below will download the filtered results.