Laboratory Accreditation for Analyses of Foods Program
About the Program
The FDA Food Safety Modernization Act (FSMA) final rule on Laboratory Accreditation for Analyses of Foods (LAAF) establishes a laboratory accreditation program for the testing of food in certain circumstances. Under the LAAF program, FDA will recognize accreditation bodies that will accredit laboratories to the standards established in the final rule (referred to as LAAF-Accredited Laboratories).
Important Note:
FDA has determined that sufficient LAAF-accredited laboratory capacity has been reached for two import testing circumstances related to specific analytes as listed on the LAAF Dashboard: in support of admission of an article of food under section 801(a) of the FD&C Act (21 CFR 1.1107(a)(4)); and to support removal from an import alert through successful consecutive testing (21 CFR 1.1107(a)(5)). See Federal Register Notice.
Although the LAAF program is being implemented for these specific import testing circumstances, sufficient laboratory capacity has not been reached for all analytical testing areas related to imported food. “The LAAF-Compliance Dates” table below lists the specific analytes for which food testing must be conducted by a LAAF-accredited laboratory, the compliance date, and additional references, where applicable, such as pertinent import alerts. Imported food testing for analytes or analyte groups not listed in this table do not require the use of a LAAF-accredited laboratory at this time.
For additional information and guidance, see FSMA Final Rule on Laboratory Accreditation for Analyses of Foods (LAAF).
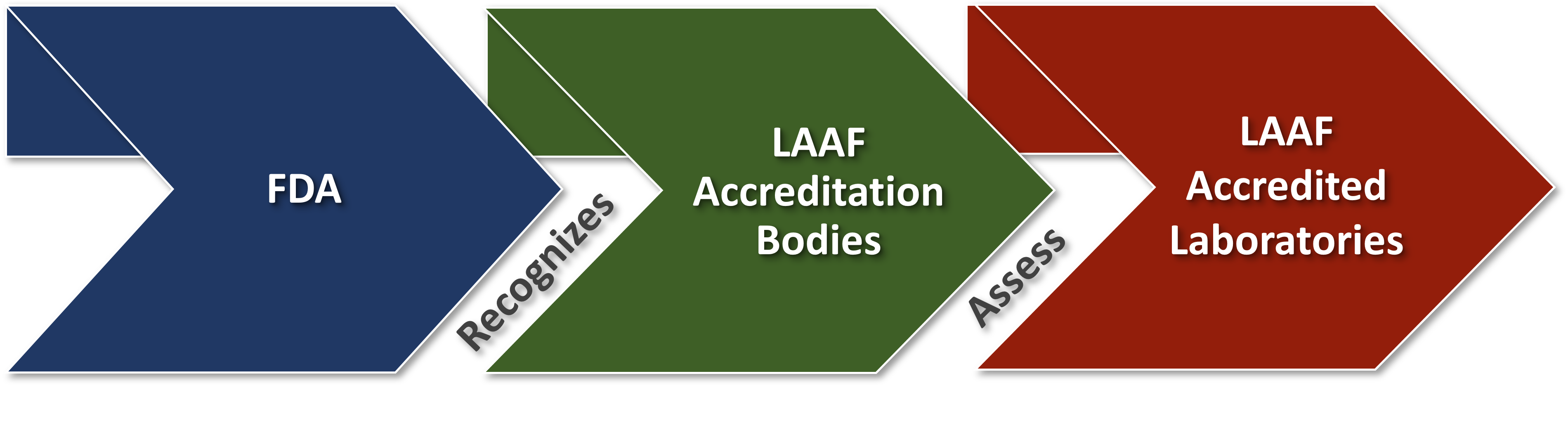
The tables below identify specific analytes for which sufficient laboratory capacity has been attained, accreditation bodies that have been recognized, laboratories that have been accredited under the LAAF program, and a list of accredited methods including analytes. One recognized accreditation body (RAB) may LAAF-accredit many laboratories. A recognized accreditation body may issue one or many certificates of accreditation for any LAAF-accredited laboratory (LAAF-AL) location covering one or multiple laboratory scopes (LS). Separate laboratory locations under common ownership may be LAAF-accredited by different recognized accreditation bodies.
The “LAAF Compliance Dates” table includes the date FDA determined laboratory capacity was sufficient and the "Compliance Date" is six months after the date laboratory capacity was reached. The compliance date is the date by which owners and consignees must begin using a LAAF-accredited laboratory for testing with data package submission directly to the FDA. The "Reference(s)" column lists impacted circumstances, such as import alerts, to assist owners and consignees.
LAAF-Compliance Dates
If filters are applied on this page, the ‘Filtered Data’ link below will download the filtered results.
Recognized Accreditation Bodies
Accreditation bodies with RAB Type of Change values of “Recognized”, “Reinstatement”, or “Probation” are considered active RABs. RABs with any other RAB Type of Change value are considered inactive. Active RAB Count is the distinct number of active RABs after the data is reduced by any applied filters. Inactive RABs are not included in the total and displayed in red or blue with strikethrough. RABs with RAB Type of Change values that are “Revocation of Recognition”, or “Denial of Renewal of Recognition” are displayed in red with strikethrough. RABs that are voluntarily withdrawn from LAAF program will have RAB Type of Change value “Relinquishment of Recognition’ and are displayed in blue with strikethrough.
If filters are applied on this page, the ‘Filtered Data’ link below will download the filtered results.
LAAF-Accredited Laboratories
LAAF-accredited laboratories that have at least one scope with LS Type of Change value of “Approved Scope”, “Reinstated”, or “On Probation” are considered active LAAF-accredited laboratories. Laboratories with none of these LS Type of Change values are considered inactive. Active LAAF-AL Count is the distinct number of active LAAF-ALs after the data is reduced by any applied filters. Inactive LAAF-ALs are not included in the total and are also displayed in red or blue with strikethrough. Inactive scopes are also displayed on the LAAF-Accredited Laboratory Scopes table in red or blue with strikethrough.
If filters are applied on this page, the ‘Filtered Data’ link below will download the filtered results.
LAAF-Accredited Laboratory Scopes
LAAF-Accredited Laboratory scopes with LS Type of Change values that are “Approved Scope”, “Reinstated”, or “On Probation” are considered active. Laboratory scopes with any other status are considered inactive. LAAF-AL scopes with LS Type of Change values that are “Suspended”, “Withdrawn”, or “Disqualified” are displayed in red with strikethrough. LAAF-AL scopes that are voluntarily withdrawn from LAAF accreditation will have LS Type of Change value “Relinquishment of LAAF-accreditation" and are displayed in blue with strikethrough.
If filters are applied on this page, the ‘Filtered Data’ link below will download the filtered results.